¥0
询价
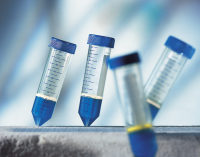
OncoQuick分离管
0 人参与
试用规则
提交申请

商家审核

联系发货

提交报告
试用说明
1、试用品规格:2个/包; 2、运费: 包邮 ; 3、联系时间: 无; 4、申请要求: 无; 5、其他说明: 无;
图文详情
产品属性
OncoQuick分离管
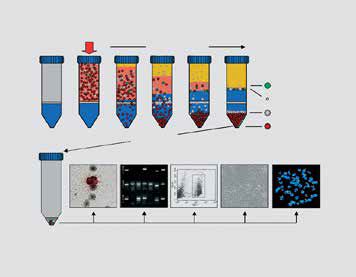
OncoQuick循环肿瘤细胞分离管能够从人的外周血高效快速分离并富集循环肿瘤细胞。该试剂盒的利用细胞密度梯度原理进行分离,具有快速、可重复、节省成本的特点,同时其回收率与免疫磁珠法一样高。
OncoQuick循环肿瘤细胞分离管内置微孔隔板,下面是蓝色的分离液。非常适用于富集从上皮实体肿瘤释放的循环肿瘤细胞。
OncoQuick循环肿瘤细胞分离管内置微孔隔板,下面是蓝色的分离液。非常适用于富集从上皮实体肿瘤释放的循环肿瘤细胞。
- 操作过程大约需要45分钟
- 可重复的回收率 >70%
- 去除红细胞可达6个数量级
- 无需特别的实验室设备
- 无需磁珠
- 不会封闭生物标志物分子
- 直接从全血富集
注意:OncoQuick循环肿瘤细胞分离管仅限于科研用途!
订购信息:

该产品被引用文献:
[1] Telomerase - A Suitable Marker for Circulating Tumor Cells in Peripheral Blood of Melanoma Patients. Poster presented at ASCB 2000, San Francisco (USA).
[2] National Committee for Clinical Laboratory Standards (NCCLS): Procedure for the Collection of Diagnostic Blood Specimens by Venipuncture; 4th ed., Approved Standard, H3-A4, National Committee for Clinical Laboratory Standards (NCCLS), Villanova, PA, 1998.
[3] National Committee for Clinical Laboratory Standards (NCCLS): Procedures for the Handling and Processing of Blood Specimens; Approved Guideline, H18-A, National Committee for Clinical Laboratory Standards (NCCLS),Villanova, PA, 1990.
[4] National Committee for Clinical Laboratory Standards (NCCLS): Protection of Laboratory Workers from Instrument Biohazards and Infectious Disease Transmitted by Blood, Body Fluids, and Tissue; Approved Guideline, M29-A, National Committee for Clinical Laboratory Standards (NCCLS), Villanova, PA, 1997.
[5] The Occupational Safety & Health Administration (OSHA): Final Standard for Occupational Exposure to Bloodborne Pathogens. 56 Fed. Reg. 64 175, Dec. 6, 1991; 29 CFR Part 1910.1030.
[6] Centers for Disease Control (CDC): Update: Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and Other Bloodborne Pathogens in HealthCare Settings. MMWR 1988, 37 No. 24, p. 380.
产品属性
库存:大量
品牌:greiner bio-one
产地:德国
供应商:格瑞纳生物科技(上海)有限公司
货号:227250