文献支持Ames试验试剂盒Ames MPF Penta 1 - 1 Sample Ames Test Kit with 5 Strains
价格: 询价
产品详情
文献和实验
相关推荐
库存 :请询
英文名 :Ames MPF Penta 1 - 1 Sample Kit with 5 Strains
CAS号 :N/A
保质期 :请询
供应商 :青木生物技术(武汉)有限公司
保存条件 :请询
Unit5 x 48 Measuring Points: 1 Sample per Strain if tested in Triplicates, 6 Dilutions, +/- 1254 Aroclor-induced S9, Negative and Positive Controls
Description
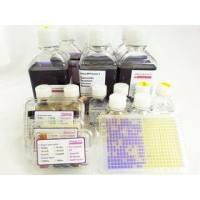
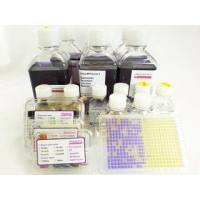
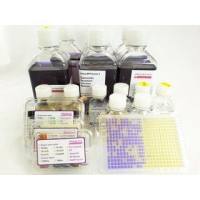
Ames MPF Kit is a liquid microplate fluctuation assay kit, standardized and quality controlled. It is a ready to use Kit for the Ames mutagenicity assay and includes 10 vials each of semisolid TA98, TA100, TA1535, TA1537, E.coli WP2 uvrA, E.coli WP2 pKM101, Booster, Growth -, Exposure -, Reversion Indicator Media, Positive Controls, Post-Mitochondrial Supernatant S9 from lyophilized Aroclor 1254 induced rat liver. The assay is using the same strains, positives controls and rat liver S9, cofactors as listed in OECD 471 and is quality controlled according to OECD 471. The liquid media used in this assay allow to test successfully pharmaceuticals, (agro-)chemicals, cosmetics, water, soil, many publication are referenced on this homepage. Especially volatile compounds, or genotoxic impurities available in low quantities, e.g. nitrosamines can be tested successfully with the Ames MPF.
Characteristics
- Quality controlled kit including all strains, S9, media, positive controls - no inhouse quality control, no sterility control are necessary
- Certificate of Analysis is provided
- Excel Calculation Sheet to support data Interpretation free of charge
- Compliant with ICH M7 allowing testing for very small quantities of genotoxic impurities, e.g. nitrosamines
- Suitable for volatile test compounds
- Number of tests corresponds to 5 x 480 agar plates.
- Standardized procedure
- Shipped at room temperature from external storage in Germany or from Switzerland
- High sensitivity and excellent correlation with agar plate test (see referenced publications)
- 4 times less test compound as compared to the agar plate test, optimized for screening purposes
- Less hands-on-time and simultaneous processing of several replicates
- Automation available - high throughput
- 3Rs - 12 fold less consumption of rat liver liver S9, significant less test animals sacrificed
- Less error prone due to easy colorimetric read-out
- Waste - Significant less plastic waste and thus reduced contaminated waste in Environment

青木生物技术(武汉)有限公司
代理商实名认证
钻石会员
入驻年限:5年